

 News
News Industry News
Industry News Drunk driving is an extremely dangerous and socially harmful behavior. Alcohol's effects on the human body are complex and can severely impair a driver's reaction speed and judgment while driving. To effectively prevent drunk driving, breathalyzers have become a crucial tool for law enforcement. Have you ever wondered how a breathalyzer works? How does it identify a driver's blood alcohol content? Before we delve into this, let's first discuss the effects of alcohol on the human body.
Alcohol's Effects on the Human Body
Alcohol, chemically known as ethanol (chemical formula C₂H₅OH), is a colorless, volatile liquid with a distinctive aromatic odor. When people consume alcoholic beverages, it is absorbed through the digestive tract and distributed throughout the body through the bloodstream, particularly to the brain, liver, and kidneys. Alcohol has a depressant effect on our central nervous system, with the following main effects:
Reduced Reflexes: Alcohol slows the brain's response to external stimuli, causing the driver to react more slowly. For example, while the vehicle is moving, the driver may be unable to brake or swerve in time to respond to an emergency.
Impaired Judgment: Alcohol's effects on the prefrontal cortex can impair judgment, making it easier for people to overestimate their driving abilities or overlook dangerous situations. This cognitive bias is one of the main causes of drunk-driving accidents.
Impaired Coordination: Alcohol impairs the driver's hand-eye coordination, leading to operational errors. This effect is particularly noticeable when driving at high speeds or in complex road conditions.
Fatigue and Drowsiness: Alcohol consumption can also exacerbate driver fatigue or drowsiness, further increasing the risk of accidents.
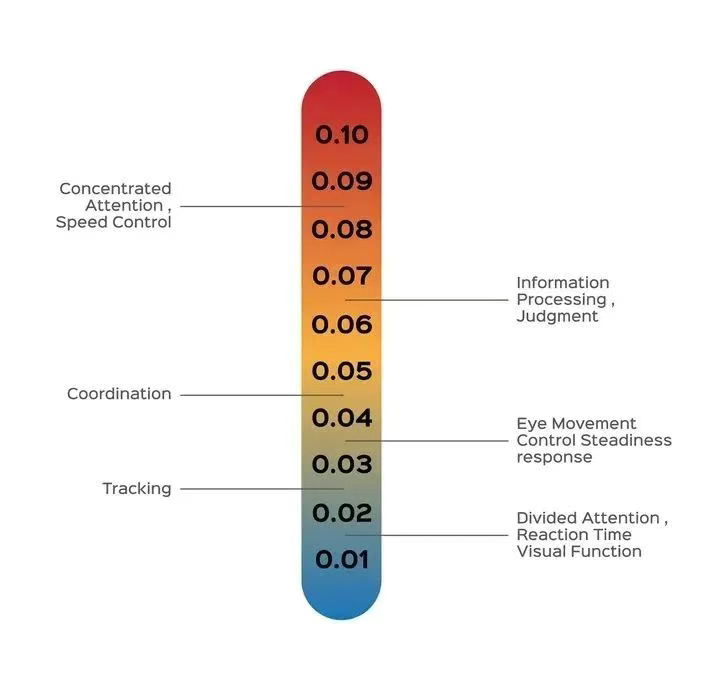
Effects of blood alcohol concentration on driving
Studies have shown that when a blood alcohol concentration (BAC) reaches 0.02%, a driver's reaction time and judgment begin to be affected. When the BAC reaches 0.08%, the driver's judgment and ability to control the vehicle are severely impaired.
How a Breathalyzer Works
A breathalyzer is a portable device used to measure a driver's blood alcohol content. It typically uses a breath sample to estimate the driver's blood alcohol concentration. The core operating principle of a breathalyzer relies on the volatility and specific chemical reactions of alcohol. Currently, the following types of breathalyzers are common:
1.Fuel Cell Alcohol Detector
Fuel cell alcohol detectors are a widely used alcohol testing tool. Their operating principle is based on the oxidation reaction of ethanol at the anode of a fuel cell. When a person exhales alcohol-containing breath, the alcohol molecules in the breath enter the fuel cell. At the anode of the fuel cell, the ethanol oxidizes with oxygen to produce acetaldehyde, releasing electrons. These electrons flow through an external circuit, generating an electric current. The magnitude of this current is proportional to the ethanol concentration. By measuring the current intensity, the alcohol detector can estimate the alcohol content in the breath.


MQ-E2-C2H5OH Electrochemical Alcohol Sensor
2.Semiconductor Alcohol Detector
Semiconductor alcohol detectors use tin oxide (SnO₂) semiconductor material, which changes its conductivity when exposed to alcohol molecules. Specifically, as exhaled breath passes through the detector, the alcohol molecules in the breath react with the semiconductor surface, changing its resistance. The detector measures this change in resistance to deduce the alcohol concentration. While this type of detector is relatively inexpensive, it is susceptible to interference from other volatile chemicals and has lower accuracy than fuel cell-based detectors.

3.Infrared Spectroscopy Breathalyzers
Infrared spectroscopy breathalyzers measure breath alcohol concentration by analyzing the absorption of specific wavelengths of infrared light by alcohol molecules. When alcohol molecules absorb infrared light of a specific wavelength, their vibrational and rotational energy levels change. The more infrared light absorbed, the higher the alcohol concentration in the breath. This method offers high accuracy, but due to the complexity of the equipment, it is typically used in laboratories or fixed testing stations.
Relationship between Breath Alcohol Concentration and Blood Alcohol Concentration
Breath alcohol testing devices measure breath alcohol concentration (BrAC), while traffic regulations typically limit the concentration to blood alcohol concentration (BAC). While the two aren't exactly equivalent, there is a certain relationship. Typically, the ratio of breath alcohol concentration to blood alcohol concentration is approximately 1:2100. This means that for every milliliter of alcohol exhaled, there are approximately 2100 milliliters of alcohol in the blood.
This ratio is based on human physiology and metabolic processes. Alcohol enters the lungs through the bloodstream and partially evaporates into exhaled air. To ensure accurate test results, law enforcement officers typically conduct multiple breath tests or request additional blood tests if the breath test result is abnormal.
It's important to note that breath alcohol concentration measurements are affected by a variety of factors. For example, the depth and duration of exhalation, body temperature, and other factors can all influence test results. To improve accuracy, modern breathalyzers typically perform multiple tests and automatically calibrate the results based on environmental factors.